As a new method of individualized tumor immunotherapy, CAR-T cell therapy has shown great therapeutic potential. However, commercial operation means new challenges. Compared with traditional chemicals, "living" biopharmaceuticals have greater uncertainty, potential problems, and risks, Pharmaceutical companies must first formulate a reasonable set of testing specifications to ensure the safety and effectiveness of CAR-T products.Today, let's take a look at how to ensure the quality of CAR-T products and better benefit patients
1. CAR-T cell amplification ability in vitro
CAR-T cell therapy requires first obtaining T lymphocytes from the patient's body, and then transduce CAR targeted domains through in vitro transgenic technology. This process needs to be fully amplified in the cell culture system to obtain enough therapeutic CAR-T cells. Therefore, the in vitro amplification ability of CAR-T cells directly determines whether the number of cells can be obtained to meet the needs of treatment. We can evaluate the in vitro amplification ability of CAR-T cell products through the cumulative number of cells obtained through multiple generations of culture, the increase of cell amplification, and the culture time required to set the number of cells [1]. These indexes reflect the proliferation and vitality of CAR-T cells, and are one of the key performance indexes for the development of CAR-T cell technology.
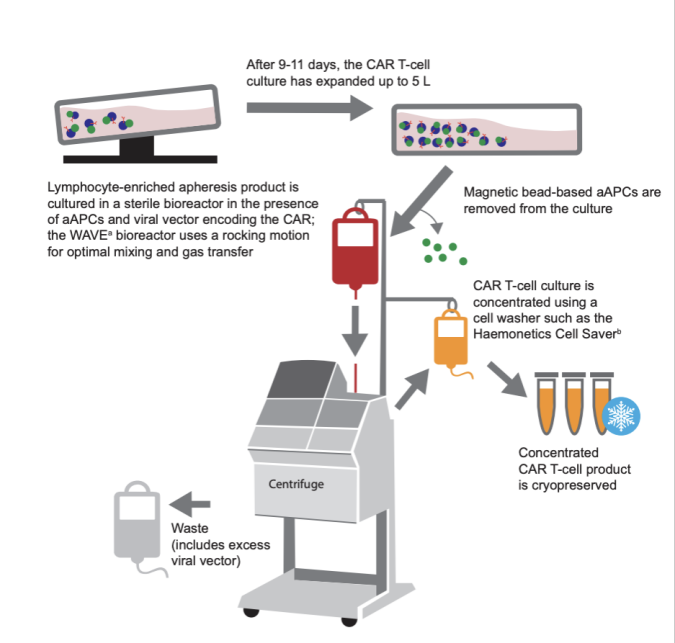
Fig1: CAR-T cell amplification process in vitro
2. Persistence of CAR-T cells in vivo
The survival time and persistence of CAR-T cells after infusion are also important indicators of function. Long-lived CAR-T cells can play a more lasting tumor killing effect. We usually regularly detect changes in the number of CAR-T cells in peripheral blood through flow cytology to evaluate their persistence in vivo. Generally, it can be detected continuously 1-6 months or more after infusion. The peak detection usually occurs 1-2 weeks after infusion. It is normal for the number of CAR-T cells to decrease over time. The ideal CAR-T cell formulation should have survival time of at least 3-6 months in vivo, only in this way can ensure a satisfactory therapeutic effect.
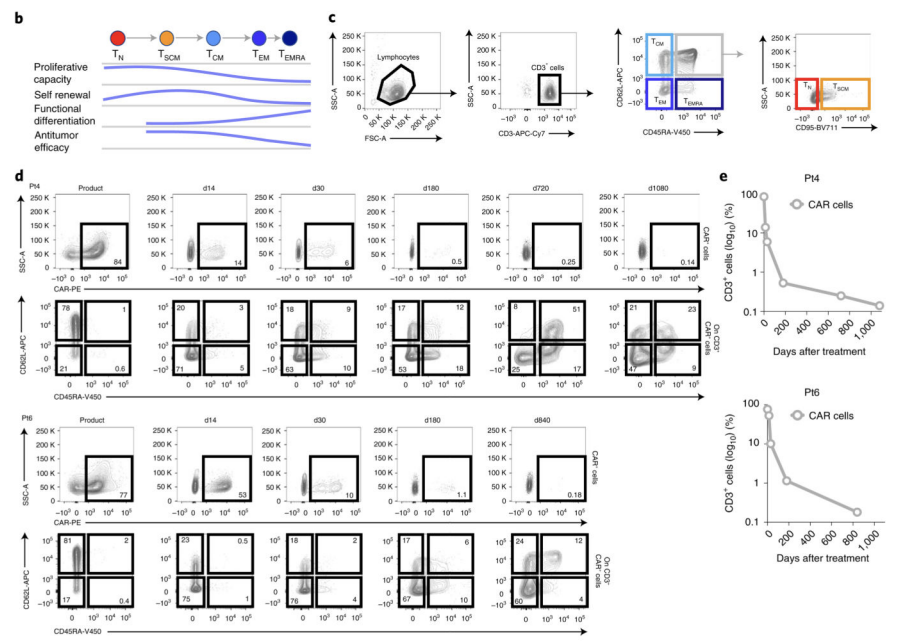
Fig2: Persistence-related detection of CAR-T cells in vivo
3. Tumor targeting performance of CAR-T cells
The key mechanism of CAR-T cell therapy is that T lymphocytes have been obtained high affinity for the ability to identify tumor surface-specific antigens after modification, so CAR-T cell products must explicit have the ability that to target tumor cells. We can detect the killing effect of CAR-T cells on tumor cell lines expressing targeted antigens through in vitro to judge their targeting performance. In addition, we can also detect the infiltration of CAR-T cells in the tumor tissue through flow cytometry in the patient's body to see if it can efficiently enter the tumor site to play a killing function [3]. The ideal CAR-T cells should be highly enriched in the tumor.
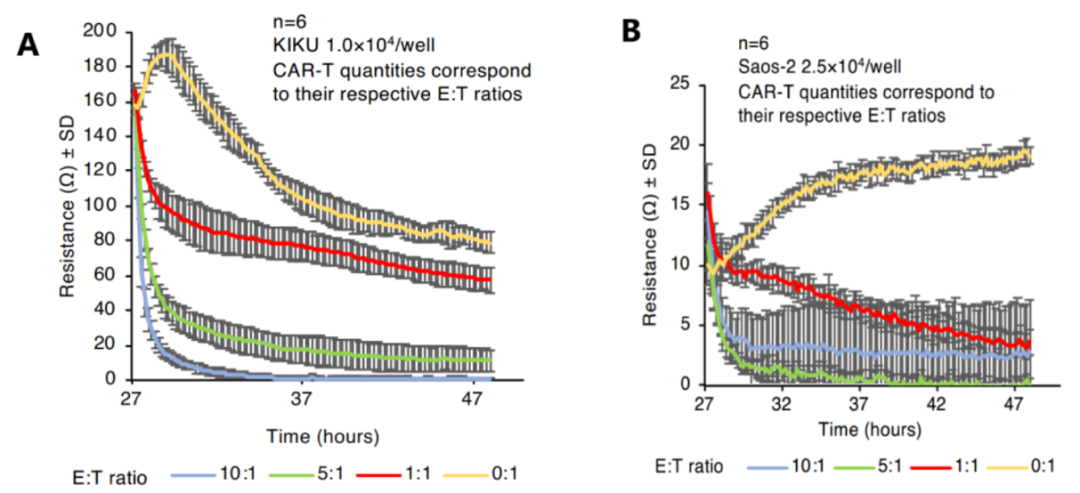
Fig3: CAR-T cell impedance detection
4. Cytotoxic activity of CAR-T cells
After CAR-T cells recognize and activate tumors, they will release various cytokines and cytotoxic particles to attack and kill tumor cells. Therefore, we can detect the levels of IFN-γ, TNF-α, perforin, granular enzyme and other molecules released by CAR-T cells to evaluate the cytotoxic activity and activation status of killing tumors [4]. In vitro, we can detect the content of the above cytokines and toxins by co-cultured CAR-T cells and target tumor cells. The degranulation experiment can also intuitively reflect the level of CAR-T cells releasing perforin and granulase, which are important indicators to evaluate the killing activity of CAR-T cell products.
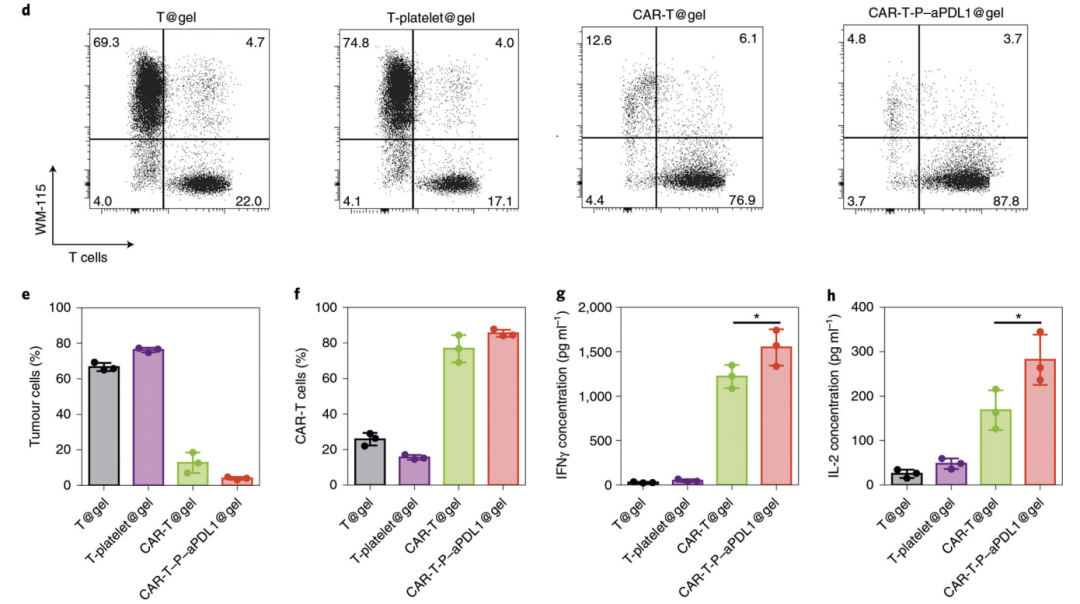
Fig4: Detection of the ability of CAR-T cells to kill target cells
5. Memory ability of CAR-T cells
Ideal CAR-T cells should also have memory function,that is mean they can achieve stronger activity after repeated activation and respond to tumor signals faster [5]. We can detect the memory effects of CAR-T cells by stimulating them multiple times in vitro, such as increase level of cytokine release and enhance of killing activity. After this CAR-T cell with memory ability is infused into the body, it can achieve a longer-lasting and stronger killing effect on tumors.
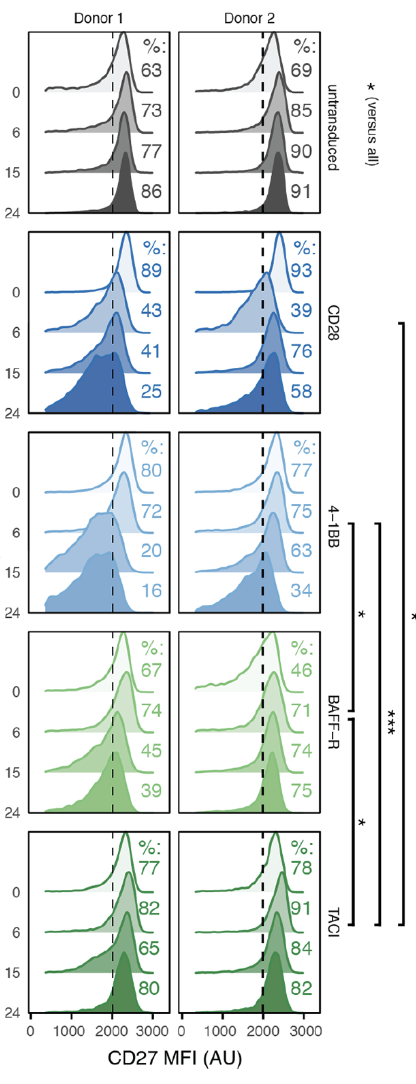
Fig5: Long-term persistence detection of CAR-T cells
6. Safety evaluation of CAR-T cell therapy
The mainly risk of CAR-T cell therapy is that may cause severe cytokine release syndrome. So we need to detect the levels of CRS-related cytokines such as IL-6, IL-10 and IFN-γ produced by CAR-T cells, and assess the risk that CAR-T cell therapy may cause CRS. In addition, it is necessary to monitor other possible toxic side effects.
These indicators can fully reflect the key performance of CAR-T cells, including proliferation ability, tumor targeting, cytotoxic activity, persistence and safety. Only CAR-T cell products that meet the above requirements and indicators simultaneously can they play a real anti-tumor effect in clinical practice. Therefore, it is necessary to establish a scientific and reasonable quality evaluation system and remove obstacles to the development of ready-to-use products for developing of high-activity and high-quality CAR-T cell products, so as to achieve the long-term goal that reduce costs and improve treatment effectiveness in the future,and to benefit more patients in need of treatment.
Star product recommendation
As the most popular star therapy at present, CAR-T therapy has been approved for listing with the third CAR-T product in China. Relevant CAR-T enterprises and platforms have accelerated the pace of research and development and actively focused on differentiated layout. Some enterprises beside compete for product safety performance, it is imperative for relevant enterprises to reduce costs and increase efficiency, and domestic substitution will also become the main way for industrial development. Immune magnetic beads are important raw materials for the preparation of CAR-T cells,and play a very important role in separation and activation process of T cells. Tonglihaiyuan GMP level product ActSep ® CD3/CD28 Separation & Activation Magnetic Beads(item number: GMP-TL603) integrates separation and activation functions to achieve the separation, activation and amplification of T cells efficiently. It is suitable for related applications of human T cells, CAR-T and other T-cell culture. Moreover, and the product has completed the DMF Type II filing with the US FDA (filing number: 038124), supporting the registration and declaration of cellular drugs.
ActSep® CD3/CD28 Separation & Activation Magnetic Beads
1.T-cell exhaustion level
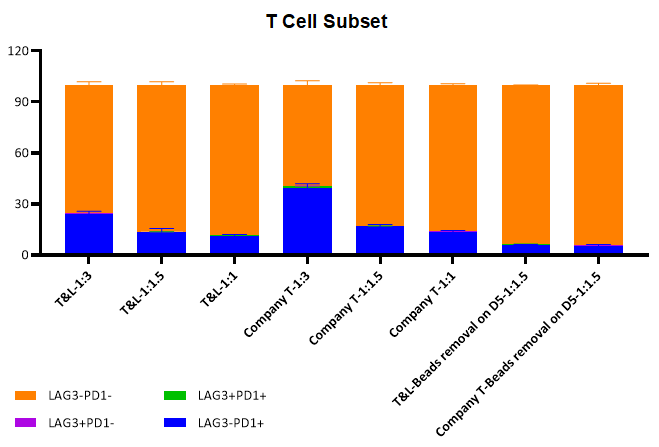
The expression levels of LAG3 and PD1 are detected after T-cell activation culture 14 days with different magnetic bead addition ratios. ActSep® is basically the same as that of competitors. The expression of ActSep ® exhaustion index is lower under the ratio of 3:1 magnetic beads to cell. In addition, removing magnetic beads on Day 5 will further reduce the exhaustion level of T cells.
2. T-cell subtype analysis
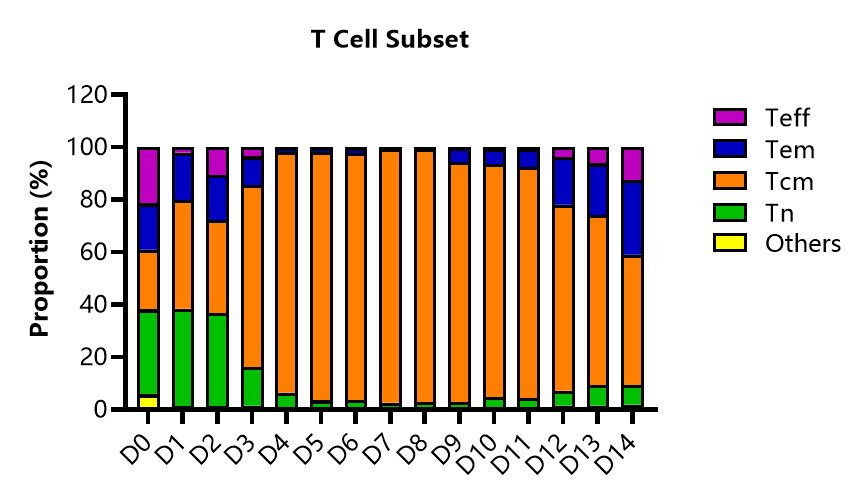
Detect the changes of T-cell subtypes at different culture points after ActSep® separation activation. The proportion of Tcm increases during the cultivation process, and the ratio of Teff to Tem increases with the extension of the cultivation time.
About T&L
T&L Biotechnology Ltd., founded in 2011, focuses on the research and development of upstream GMP grade raw materials and reagents of cell and gene therapy (CGT). We commit to providing reliable products and services for life science
Monday to Friday
09:00-17:30