Cell therapy has developed rapidly in recent years in pharmaceutical research and development, and is a new drug development model that has shown great potential in the treatment of diseases such as cancer, infectious diseases, and autoimmune diseases. On June 30th this year, the National Medical Products Administration (NMPA) of China approved the launch of the Equecabtagene Autoleucel Injection , jointly developed by Nanjing Reindeer Biotechnology and Innovent Biologics, Inc, for the treatment of adult patients with recurrent/refractory multiple myeloma,it became the first approved BCMA CAR-T therapy in China and the third approved CAR-T cell therapy product after Axicabtagene Ciloleucel Injection and Relmacabtagene Autoleucel Injection.
Since the world's first CAR-T product was approved for launch in 2017, the global cell therapy market has rapidly expanded, increasing from $10 million in 2017 to nearly $2.7 billion in 2022. According to Frost&Sullivan's forecast, the global CAR-T cell therapy market sales will reach $21.8 billion by 2030, with a compound annual growth rate of 34.8% from 2021 to 2030. Up to now, 9 CAR-T products have been launched globally, and CAR-T therapy has shone with its excellent therapeutic effect in treating various malignant tumors. However, due to various factors such as cost, research and development, production, and efficacy, CAR-T products are usually priced at over one million yuan, and high pricing limits their application, resulting in poor patient accessibility.
The high cost of CAR-T products is closely related to their high production costs, with reagents, consumables, and depreciation of fixed assets constituting the main costs. With the expansion of production scale, the cost of fixed assets is gradually diluted, and the proportion of reagent and consumables costs continues to rise. Meanwhile, key raw materials have long been monopolized by foreign enterprises, leading to problems such as long supply times and high prices, which directly threaten the rapid development of the domestic cell therapy industry. Therefore, cost reduction, efficiency improvement, and domestic substitution have become hot topics in the industry.
New product launch NanoSep™ series nanoscale cell sorting magnetic beads
T&L has been focusing on the research and production of upstream core reagent materials for cell and gene therapy (CGT) for more than ten years, and has launched NanoSep with great emphasis ™ Series of cell sorting magnetic beads, with a size of 50nm and performance comparable to imported brands, can be used for T cell enrichment or removal sorting, helping CGT customers accelerate the pace of domestic substitution and win market opportunities.
product features
50nm size / high safety, biodegradable / high stability and efficient sorting of target cells / can provide RUO level and GMP level / spot supply
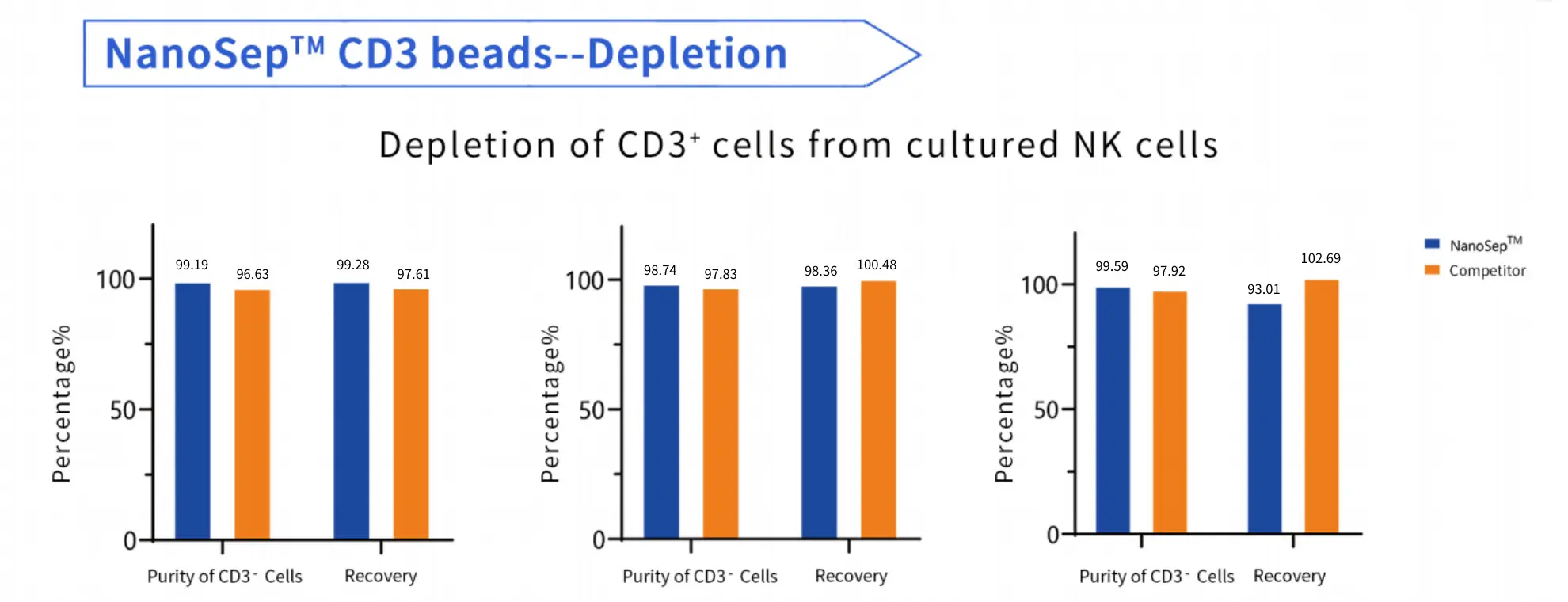
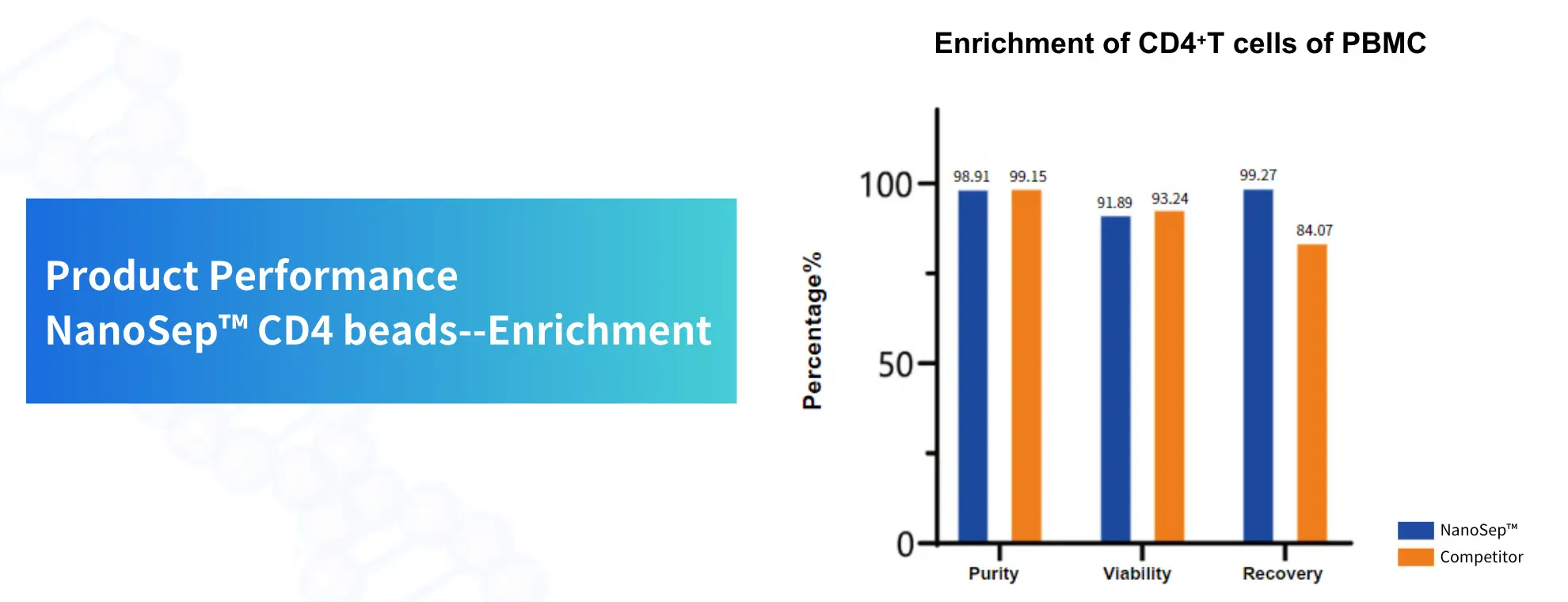
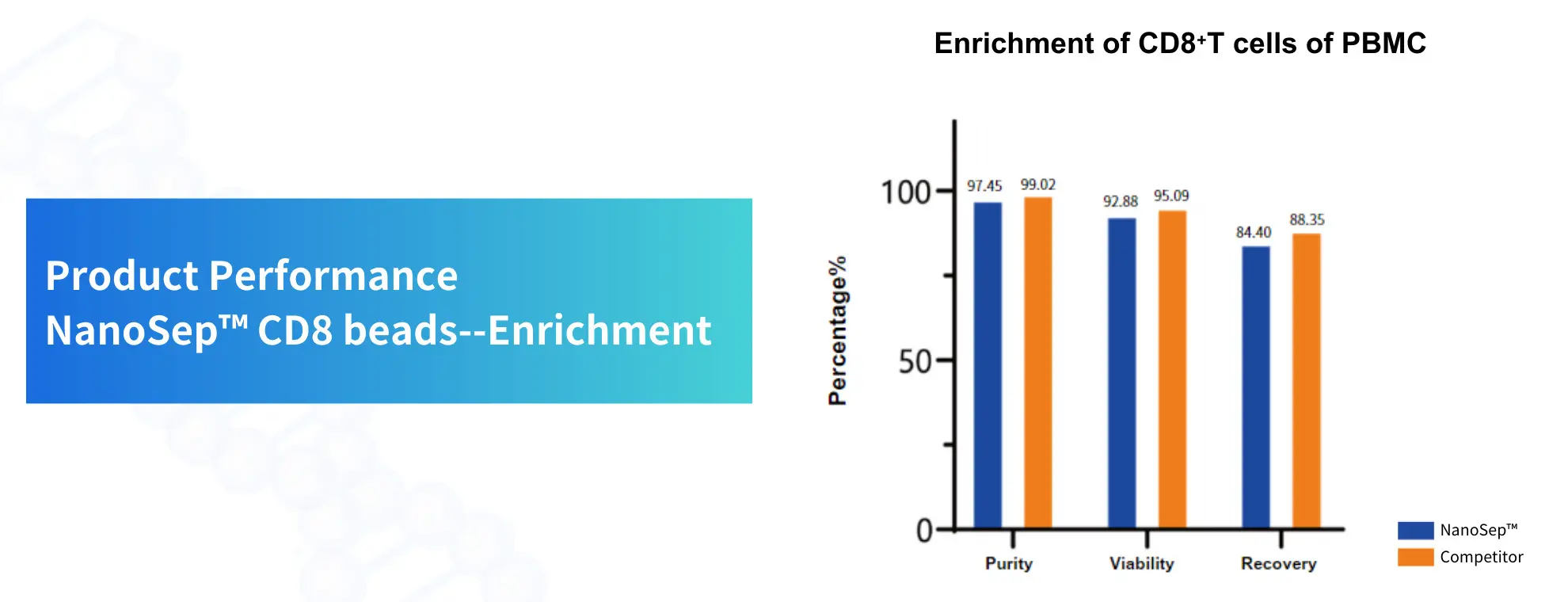
product performance
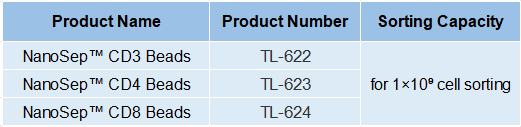
Product Information
About T&L
T&L Biotechnology Ltd., founded in 2011, focuses on the research and development of upstream GMP grade raw materials and reagents of cell and gene therapy (CGT). We commit to providing reliable products and services for life science
Monday to Friday
09:00-17:30