Recently, Beijing T&L Biotechnology Ltd. (hereinafter referred to as "T&L") received a confirmation letter from the US Food and Drug Administration (hereinafter referred to as "FDA"), stating that the company's key raw materials for cell therapy drugs, CD3 monoclonal antibody, and T cell sorting activation magnetic beads, have officially completed the DMF type II filing with the US FDA.
In the future, customers using products related to T&L can directly reference the DMF registration number in the documents submitted to the FDA for new drug registration, greatly reducing product review and evaluation time, simplifying the IND application process, and accelerating the project application process of related drugs.
Let's learn about the key information of DMF filing materials together
The Drug Master File (DMF) is an archived document submitted to the FDA for review, which includes detailed information on the production facilities, process flow, quality control, raw materials, packaging materials, and other processes used in the production, processing, packaging, and storage of drug products for human use.
The US DMF filing is a regulation made by the US FDA to maintain the confidentiality of information in DMF declarations,its main objective is to support FDA regulatory requirements and demonstrate that the product quality, safety, and effectiveness of raw material suppliers have obtained the required ratings. Raw material suppliers can directly submit confidential information related to the product to the FDA without disclosing it to their customers. However, manufacturers may have to disclose certain parts of the DMF to their customers, such as product specifications and general information, as such information is essential for product development and quality control related activities. Suppliers with a large number of DMFs are generally considered more reliable in terms of quality, regulatory status, and ability to meet cGMP requirements.
DMF Filing accelerates your drug declaration process
Usually, before a drug is launched, the applicant must submit a series of applications to the FDA, such as Clinical Research Application (IND), New Drug Registration (NDA), and Biological Product License Application (BLA), and provide all information about the drug's safety, efficacy, and quality, which involves technical content related to raw materials and energy,however, preparing these materials undoubtedly requires a lot of time and effort, therefore, it will seriously affect the process of clinical application.
The DMF filing system can solve this problem,raw material suppliers directly submit the required technical content to the FDA for filing and obtain a filing number in the form of DMF documents.,drug applicants can use the DMF filing number directly instead of providing specific information about raw materials and auxiliary materials during the application process,this can not only save approval costs, improve approval efficiency, and ensure traceability of supervision and inspection; At the same time, it greatly shortens the registration cycle, reduces repetitive research caused by differences in registration requirements, and accelerates the drug declaration process.
If you have used products registered with T&L DMF and need to reference the DMF number, please send an email to huodong@seafrom.cn,after submitting an authorization application and confirming with you, we will provide the FDA with a DMF authorization letter.
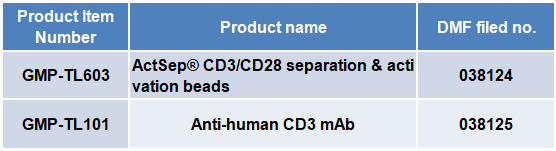
ActSepTM CD3/CD28 sorting activation magnetic beads
GMP grade CD3/CD28 sorting activation magnetic beads (Cat. No. GMP-TL603) integrate sorting and activation functions, efficiently achieving the sorting, activation, and expansion of T cells, suitable for the application of various T cell culture technologies such as human T cells and CAR-T. Time saving and efficient, pioneering in China, with excellent application performance and ultra-high cost-effectiveness.
Anti human CD3 monoclonal antibody
GMP grade anti-human CD3 monoclonal antibody (Cat. No. GMP-TL101) humanized the original CD3 monoclonal antibody (OKT3), while maintaining the OKT3 monoclonal antibody's ability to activate T cells, to the greatest extent possible, eliminating the immunogenicity of mouse derived antibodies, based on this, using CHO cells to express humanized antibodies can be better applied in the development of clinical cell drugs.
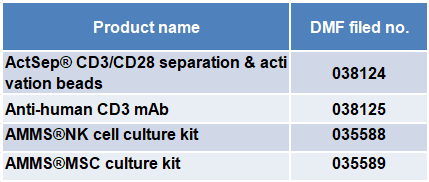
At present, T&L has completed the DMF filing of four self-developed products with the US FDA, as shown in the table below:
About T&L
T&L Biotechnology Ltd., founded in 2011, focuses on the research and development of upstream GMP grade raw materials and reagents of cell and gene therapy (CGT). We commit to providing reliable products and services for life science
Monday to Friday
09:00-17:30